New to MyHealth?
Manage Your Care From Anywhere.
Access your health information from any device with MyHealth. You can message your clinic, view lab results, schedule an appointment, and pay your bill.
ALREADY HAVE AN ACCESS CODE?
DON'T HAVE AN ACCESS CODE?
NEED MORE DETAILS?
MyHealth for Mobile
CAR T-Cell Therapy
How We Can Help You
One of the newest and most promising areas of cancer research and treatment is cancer cell therapy. Cancer cell therapy begins by collecting immune cells from your blood. Those cells are taken to a lab, modified, and returned to you to spur your immune system to more effectively target and knock-out disease.
Stanford researchers helped to develop the first FDA-approved cancer cell therapies, and the Stanford Medicine Cell Therapy Program remains at the leading edge of CAR T-cell therapy research to expand its application across more types of cancer.
What We Offer You for Cell Therapy
- Globally-recognized pioneers in cancer cell therapy research and patient care.
- Team-based treatment planning that brings together specialists from across Stanford Medicine to collaborate on your needs.
- An active clinical research program dedicated to broadening options for cancer cell therapy.
- Comprehensive support services to address your unique needs through treatment and beyond.
About CAR T-Cell Therapy
About Cell Therapy
Chimeric Antigen Therapy or CAR T-Cell Therapy is a form of immunotherapy, the newest and most promising of cancer treatments. CAR T-Cell Therapy falls into a class of immunotherapy treatment that involves genetically modifying a patient’s own immune cells to fight cancer. Stanford is a leader in the development of novel CAR T-cell treatments and continues to expand the promise of these therapies through clinical research.
Understanding CAR T-Cell Therapy
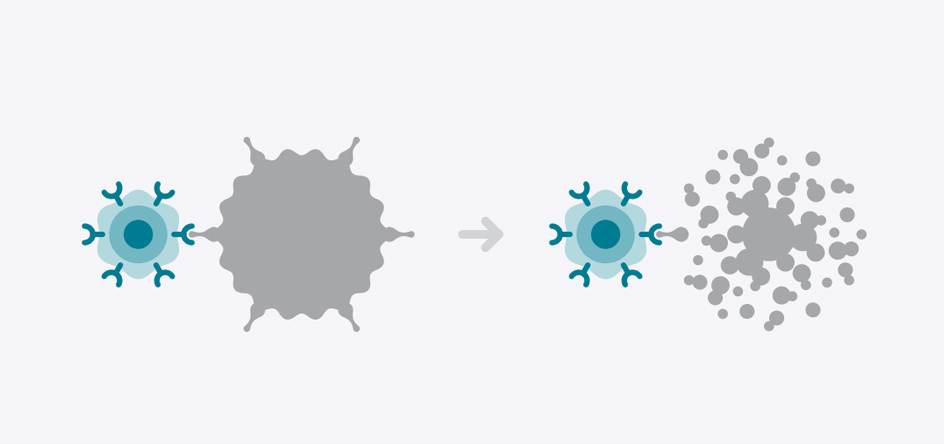
During CAR T-Cell therapy, a type of white blood cell called a T-Cell is collected and genetically modified to develop a chimeric antigen receptor or CAR. Those receptors latch onto a specific antigen on the surface of the cancer cell to disrupt and destroy it.
What You Need to Know About CAR T-Cell Therapy
CAR T-Cell Therapy is approved by the FDA and offered at Stanford for patients with specific types of non-hodgkin lymphoma and acute lymphoblastic leukemia in patients up to the age of 25.
It is available through clinical trials for other forms of cancer.
What to Expect of CAR T-Cell Therapy
Before
During
After
In your first visit, you will meet with your care team to:
- Discuss the treatment approach.
- Discuss the process, potential side effects, outcomes, and impact on your day-to-day living, activities and quality of life.
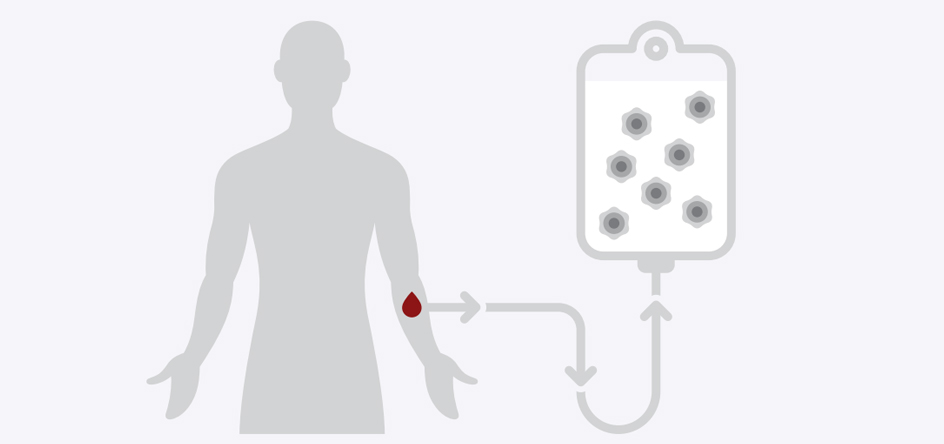
The CAR T-Cell therapy itself begins with a process similar to blood donation, except that it takes a few hours to complete.
First, we’ll run your blood through a special piece of equipment called an Apheresis machine. It helps sort your T cells from other blood and immune cells. Then, it returns the non-T cells to your body.
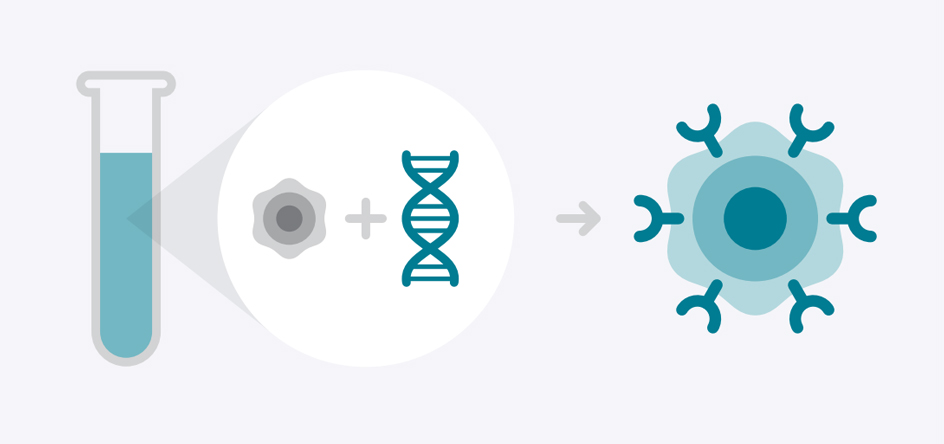
Your T cells will be sent to a lab to be genetically modified and grow chimeric antigen receptors, or CARs, on their surfaces. These CARs should enhance your immune system’s effectiveness at targeting and attacking certain cancer cells anywhere they may be hiding in your body.
After that, you will be treated with a preparative regimen of chemotherapy to prepare your body to receive the modified CAR-T cells.
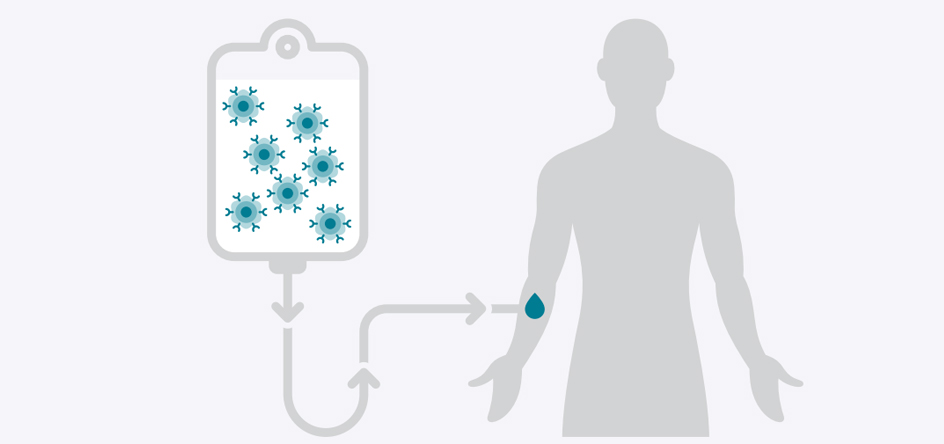
The modified CAR-T cells will be infused back to you. Most patients remain in the hospital for about 2 weeks after infusion, so your care team can keep close watch on your progress.
Recovery is influenced by several factors, including your original diagnosis, your age, your overall health, the chemotherapy you received, and when you experienced any side effects or complications during cell therapy.
About 100 days after your treatment, your care will be transitioned to your primary oncologist for regular check-ups. Your doctor will ask you how you are doing, assess your progress, and offer additional care and guidance.
In your first visit, you will meet with your care team to:
- Discuss the treatment approach.
- Discuss the process, potential side effects, outcomes, and impact on your day-to-day living, activities and quality of life.
The CAR T-Cell therapy itself begins with a process similar to blood donation, except that it takes a few hours to complete.
First, we’ll run your blood through a special piece of equipment called an Apheresis machine. It helps sort your T cells from other blood and immune cells. Then, it returns the non-T cells to your body.
Your T cells will be sent to a lab to be genetically modified and grow chimeric antigen receptors, or CARs, on their surfaces. These CARs should enhance your immune system’s effectiveness at targeting and attacking certain cancer cells anywhere they may be hiding in your body.
close Before
After that, you will be treated with a preparative regimen of chemotherapy to prepare your body to receive the modified CAR-T cells.
The modified CAR-T cells will be infused back to you. Most patients remain in the hospital for about 2 weeks after infusion, so your care team can keep close watch on your progress.
close During
Recovery is influenced by several factors, including your original diagnosis, your age, your overall health, the chemotherapy you received, and when you experienced any side effects or complications during cell therapy.
About 100 days after your treatment, your care will be transitioned to your primary oncologist for regular check-ups. Your doctor will ask you how you are doing, assess your progress, and offer additional care and guidance.
close After
Patient Guidebook
FAQs for CAR T-Cell Therapy
CAR T-Cell therapy is a type of immunotherapy, which increases the capacity of immune cells to fight disease. A type of white blood cell called a T-Cell is collected and genetically modified to develop a chimeric antigen receptor, or CAR. Those CARs latch onto a specific antigen on the surface of the cancer cell to disrupt and destroy it.
CAR T-Cell therapy is approved by the FDA to treat some forms of non-hodgkin lymphoma and for patients with recurrent or refractory acute lymphoblastic leukemia up to age 25.
There are several ongoing trials of CAR T-cell therapy for other forms of blood cancers and some solid tumors.
We work with patients and insurers to secure health insurance coverage for eligible patients. More insurers are covering care for FDA-approved therapies.
Side effects are generally easier to tolerate than those associated with chemotherapy.
Our care team will monitor your response to the treatment and manage any reactions or complications. Side effects can be mild or more severe but are treatable and generally indicate the therapy has activated the immune system to fight the cancer.
The most common side effect of CAR T-Cell therapy is called cytokine release syndrome or CRS. It can affect up to 9 in 10 CAR T-Cell patients. It is generally brief, lasting about a week. Many patients liken it to the flu with fever, low energy, and body aches. It is treatable with medication.
The other side effect is known CAR T-Cell-related encephalopathy syndrome or CRES. Its symptoms are more severe than those of CRS and may include memory loss or confusion. In rarer cases, they may affect speech. CRES is treatable. Symptoms usually go away after a few days.
Stanford has the knowledge and resources to manage these reactions when then they occur.
The treatment process involves:
- Evaluation to confirm that CAR T-Cell Therapy is a recommended treatment
- Collection and sorting of blood cells to isolate T cells for therapy
- Genetic modification of T cells in a lab to cause them to develop chimeric antigen receptors that target cancer
- Culturing these modified cells in a lab to increase the number of available CAR-T cells
- Preparative regimen of chemotherapy to get your body ready to receive the CAR-T cells
- Infusion of the CAR-T cells into the body
- Recovery of a few months, while your immune system stabilizes and gets stronger
Our Clinics

 900 Blake Wilbur Drive
900 Blake Wilbur Drive
900 Blake Wilbur Drive
900 Blake Wilbur DrivePalo Alto, CA 94304
Phone: 650-723-0822 Getting Here
Are you a patient or physician interested in cancer cell therapy? call 650-723-0822.
CAR T-Cell Therapy
Stanford doctors are pioneers in the newest and most promising area of cancer research and treatment known as cancer cell therapy.
CAR-T cell therapy
immunotherapy
monoclonal antibodies
Cancer Cell Therapy Program