Doctor Stories
Physicians Perform Stanford Health Care’s First Autologous Islet Transplants in Two Patients
For patients with chronic and debilitating pancreatic disorders, autologous islet transplantation represents a possible treatment approach that doesn’t require systemic immunosuppression. Stanford Medicine physicians recently performed successful autologous islet transplants in two patients. These were the first procedures of their kind at Stanford Health Care and a milestone for doctors, physician-researchers, and staff within the Benign Pancreas Program.
“These successful transplants mark the culmination of years of effort to establish this program,” says Walter Park, MD, associate professor of medicine in the Division of Gastroenterology and Hepatology and medical director of the Benign Pancreas Program at Stanford Health Care. “This is a major step forward in our ongoing plans to further broaden our ability to serve patients requiring these procedures.”
CARE AT STANFORD

The Benign Pancreas Program provides unparalleled experience and comprehensive care to patients with all types of pancreatitis and pancreatic cysts.
650-736-5555
Addressing an Intractable Condition
The pancreas performs critical functions required for both proper digestion (exocrine function) and regulating blood glucose levels (endocrine function). Islets comprise a cluster of cells, including insulin-producing beta cells, located in the pancreas and responsible for endocrine function. Patients with progressive forms of pancreatitis experience gradual loss of both endocrine and exocrine function, as well as increasing pain related to chronic or acute recurrent inflammation.
“Patients with chronic or acute recurrent pancreatitis experience debilitating pain related to constant inflammation and scarring of the pancreas,” explains Dr. Park. For these patients, this condition can lead to severe digestive issues, diabetes, chronic pain, and an increased risk of pancreatic cancer. For patients who are no longer responsive to medical or endoscopic symptom management, total pancreatectomy with autologous islet transplantation is a promising remaining option.
Dr. Park explains that in these cases, removal of the pancreas alleviates pain in up to 80% of patients. However, both timing of the procedure and patient selection are critical. “Although there are straightforward ways to supplement lost exocrine function, removal of the pancreas results in complete loss of endocrine function and brittle diabetes, which is extremely difficult to manage.”
Identifying the Best Candidates for the Procedure
“The best patients for these procedures are often those genetically predisposed to pancreatitis, because they tend to be younger and will likely have a more predictable course of recurrent episodes of pancreatitis in the future,” says Dr. Park. Because some mutations also predispose a patient to developing pancreatic cancer, removing the pancreas at a relatively young age can prevent pain, as well as risk of developing cancer. For this reason, such procedures can offer pediatric patients already suffering from symptoms a new lease on life.
For patients with pancreatitis, there is an optimal window of time during which islets remain sufficiently viable to support their isolation and transplantation. Therefore, early identification of good candidates is key.
“The process of determining suitable candidates is a balancing act,” says Dr. Park. “Although it’s necessary to avoid being overly aggressive in performing a procedure too early in someone that might benefit from symptom management alone, waiting too long to obtain viable islets can eliminate transplantation as an option.”
Isolating and Transplanting Islets to Reinstate Endocrine Function
For patients undergoing this procedure at Stanford Health Care, the pancreas is removed by Stanford Medicine surgeons and immediately transported to a facility at Baylor University Medical Center. There, the islets are carefully extracted and prepared for transplantation. Upon receipt of the purified islets, Stanford Medicine interventional radiologists infuse them into the patient’s liver through the portal vein, where they gradually engraft and hopefully begin producing insulin.
Despite delivery of the patient’s own cells, approximately 50% of transplanted islets will not survive the transfusion. For the islets that remain viable, engraftment can take up to three months. During this time, patients undergo constant glucose monitoring to manage appropriate insulin administration. Marina Basina, MD, a clinical endocrinologist with the Benign Pancreas Program, leads the team responsible for monitoring these patients and evaluating biomarkers that signal whether the transplanted islets are producing insulin.
The goal is to gradually wean patients off the need for exogenous insulin. However, in cases where too many islets were lost during transplantation and engraftment, balancing how much insulin is being created against what patients need to receive to avoid becoming diabetic is critical. Dr. Park advises that, in this regard, transplant success is not an all-or-nothing proposition.
“A successful procedure is one that eliminates a patient’s pain and risk of cancer,” he explains. “A home run would be for patients to be both pain free and insulin independent. However, base hits under these circumstances are certainly acceptable.”
Driving Innovative Research
Even in the best post-transplant scenarios, data show that transplanted islets eventually lose their function. “By 10 years, approximately 80% of patients require some level of exogenous insulin, even after a successful transplant.” Addressing this eventuality is an active area of research within the Stanford Medicine Institute for Stem Cell Biology and Regenerative Medicine.
Preclinical studies overseen by Seung Kim, MD, PhD, professor of medicine in the Department of Developmental Biology at Stanford Medicine, revealed that smaller islet clusters tend to survive longer and demonstrate comparable endocrine function relative to their larger precursors. His findings showed that disaggregating islets into individual cells resulted in spontaneous formation of smaller clusters that demonstrated similar or higher levels of insulin production.
These “pseudo-islets” could potentially benefit patients undergoing either autologous or eventually allogeneic islet transplantation. The California Institute of Regenerative Medicine is currently supporting efforts to transform Dr. Seung’s protocol into something that could be applied therapeutically on a larger scale.
“Our vision involves clinical trials where we extract islets, perform autologous islet transplantation, and then supplement those procedures with pseudo-islets after the primary procedure,” explains Dr. Park. The efficacy of pseudo-islets in this context would offer hope to patients with relatively few viable islets.
“In addition to expanding our infrastructure to support the islet-extraction process on-site, we are actively engaged in research that will position us as leaders among national centers that currently offer these procedures,” continues Dr. Park.
Improving Lives Through Teamwork and Collaboration
Dr. Park explains that their recent success with this technique validates the time spent building the Benign Pancreas Program into its current form. “Although we may have been equipped clinically and administratively for success prior to this, the spectrum of care required by patients before, during, and after this procedure drove a very intentional approach to building a strong multidisciplinary team.”
He emphasizes that the positive outcomes with the first two patients would not have been possible without efforts from professionals across multiple disciplines, including the following physicians and support staff:
- Varvara Kirchner, MD, Associate Professor of Surgery (abdominal transplant surgery)
- Brendan Visser, MD, Professor of Surgery (hepatobiliary surgery)
- Avnesh Thakor, MD, PhD, Associate Professor of Radiology (interventional radiology)
- Alexander Vezeridis, MD, PhD, Assistant Professor of Radiology (interventional radiology)
- Marina Basina, MD, Clinical Professor of Medicine (endocrine medicine)
- Naoko Fushimi, MS, RN, Nurse Practitioner
- Catherine McIntyre, PhD, Operations and Scientific Director, Bone Marrow Transplant and Cell Therapy (BMT-CT) Program
- Christopher Bainter, ASQ, CQPA, Senior Quality Coordinator, Cell Therapy Facility
- Aman Siddiqui, Supervisor, Cell Pharmacy, (BMT-CT)
- Christine Hartley, MBA, MSN, RN, ACNP-BC, Executive Director, Solid Organ Transplant Destination Service Line
- Thomas Cartwright, Senior Project Manager of Strategy and Operations, Department of Radiology
- Heather Packard, Director of Clinical Services, Solid Organ Transplant and VAD
“Establishing this program a decade ago announced both our willingness and ability to care for very complex chronic and acute recurrent pancreatitis patients,” says Dr. Park. “Having proven our expertise in this arena, our mission is now to find ways to constantly improve.”
Learn more about Stanford Health Care’s Benign Pancreas Program.
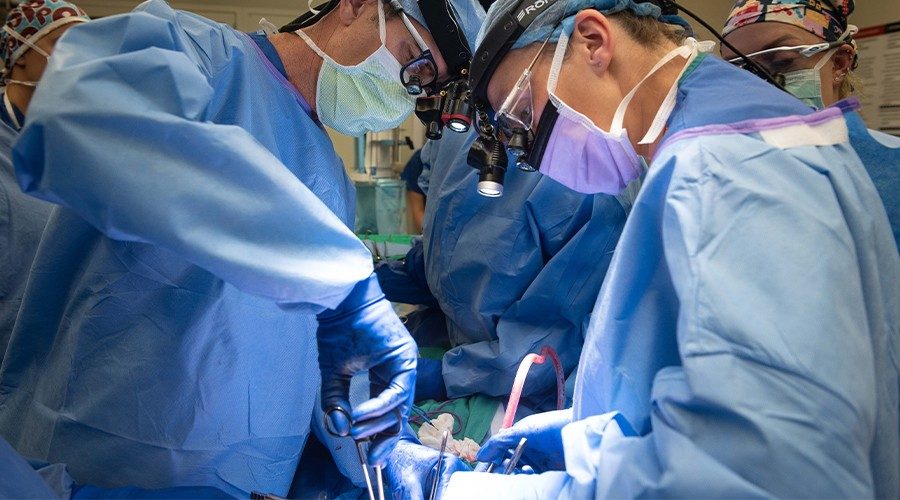
Top image of Brendan Visser, MD and Varvara Kirchner, MD (left to right) removing the pancreas.
About Stanford Health Care
Stanford Health Care seeks to heal humanity through science and compassion, one patient at a time, through its commitment to care, educate and discover. Stanford Health Care delivers clinical innovation across its inpatient services, specialty health centers, physician offices, virtual care offerings and health plan programs.
Stanford Health Care is part of Stanford Medicine, a leading academic health system that includes the Stanford University School of Medicine, Stanford Health Care, and Stanford Children’s Health, with Lucile Packard Children's Hospital. Stanford Medicine is renowned for breakthroughs in treating cancer, heart disease, brain disorders and surgical and medical conditions. For more information, visit: www.stanfordhealthcare.org.
